-
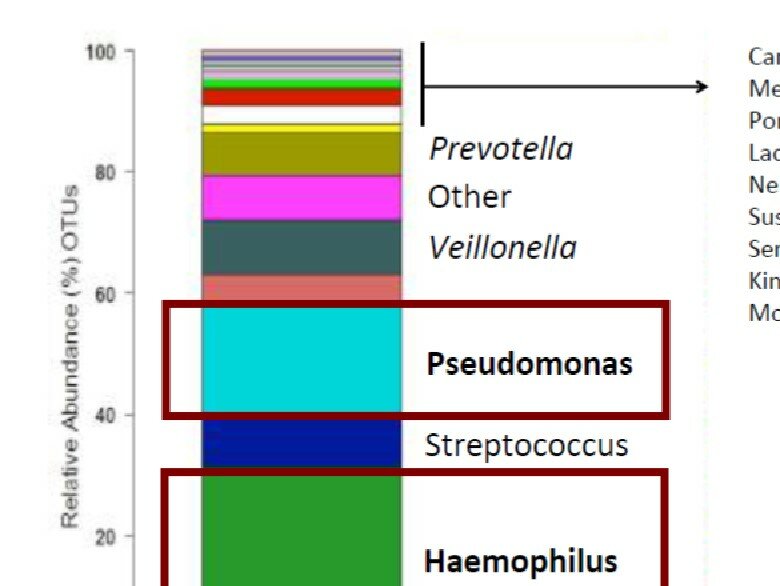
Developing the next generation of treatments requires multinational co-operation and co-ordination. Bronchiectasis has historically been a neglected "Orphan" disease. An international network will build capacity to perform high impact clinical trials and observational studies in bronchiectasis
-

The European Bronchiectasis Registry is supported by the European Union Innovative Medicines Initiative under the "New Drugs for Bad Bugs" programme, to help facilitate the development of new antibiotics against Gram-negative infections
-

EMBARC work closely with the European Lung Foundation to promote patient priorities and improve patient education and resources.
-
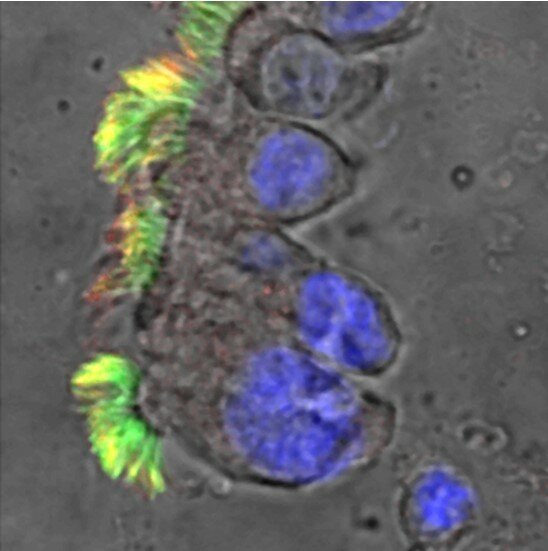
The largest bronchiectasis biobanking study is underway and will gather in depth data on cilia, inflammatory markers and many more clinical measures linked to the registry.
-
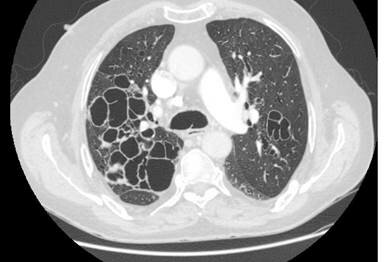
Advancing research and improving clinical care for patients with non-cystic fibrosis bronchiectasis
-

EMBARC promotes awareness and clinical excellence in bronchiectasis care through educational events, courses and online resources.
-
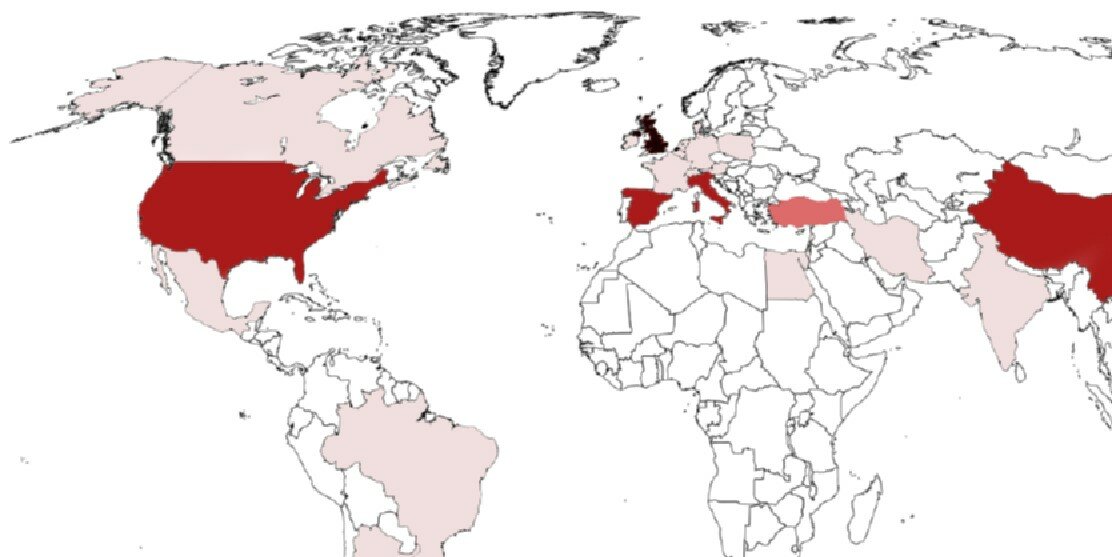
EMBARC works with the European Respiratory Society and the national societies and networks around the world to promote co-operation, data sharing and clinical research in bronchiectasis
-
EMBARC is a European Respiratory Society clinical research collaboration, dedicated to encouraging participation from young and experienced researchers around Europe in the field of bronchiectasis
EMBARC is a pan-European network committed to promoting clinical research and education in bronchiectasis, through sharing of protocols, research idea and expertise. Central to this project is the creation of the European Bronchiectasis Registry, a collaboration open to all investigators around Europe caring for patients with bronchiectasis.
Latest News
3rd European Bronchiectasis Workshop
The 3rd European Bronchiectasis Workshop (EBROW23) is being held in Milan, February 23-25th 2023. The programme is available to view online and there is still time to register your …
Read More3rd Bronchiectasis Patient Conference - 12th March 2023
Following the success of the previous two bronchiectasis patient conferences, EMBARC clinicians have continued to work with the ELF patient advisory group to develop the 3rd …
Read More5th World Bronchiectasis & NTM Conference
The 5th WBNC will be held in Prague 30th June – 2nd July 2022 with plans of a hybrid conference experience so those who cannot travel will not miss out! The call for abstracts is now open …
Read MoreLatest Research
Bronchiectasis insanity: Doing the same thing over and over again and expecting different results?
New Insights Into the Epidemiology of Bronchiectasis.
Medical management of bronchiectasis.
Recruitment
Join EMBARC
EMBARC is an open group and free to join.
For more information contact [email protected]
Sign up at the registration page
| Talk to us on Twitter! |

|
| Connect with us on LinkedIn |

|




